by R. Passov
Recently, I watched a YouTube of a talk given by Jennifer Doudna. This past May, in 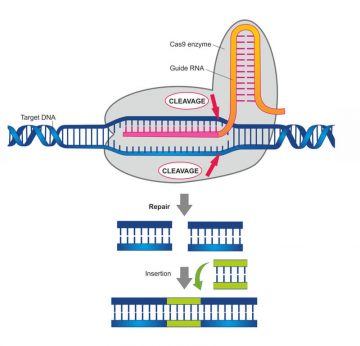 front of some her UC Berkeley colleagues, Doudna shared, “a story … about some research … that led in an unexpected direction … ” producing “ … some science that has profound implications going forward…but also makes us really think about what it means to be human and what it means to have the power to manipulate the very code of life …”
front of some her UC Berkeley colleagues, Doudna shared, “a story … about some research … that led in an unexpected direction … ” producing “ … some science that has profound implications going forward…but also makes us really think about what it means to be human and what it means to have the power to manipulate the very code of life …”
It all started, Doudna explains, when she got a call from someone at Cal who said you don’t know me but you’re doing the type of research that’s connected to my work. Her colleague had noticed that “ …many types of bacteria in their chromosomes have a sequence of DNA that is a storage site for sequences that come from viruses that infect those cells.”
“These are the CRISPERS …” a record of the DNA from all prior infections, “… a genetic vaccination card for bacteria.”
Doudna is generous with praise, never missing an opportunity to share credit. One lucky lab member collected soil samples, then sequenced DNA from bugs in those samples looking for alternative CRISPER pathways. The result uncovered different flavors of the CRISPER-Cas immune system. Which got Doudna to thinking “… about the difference in the type of CRISPER systems in nature…”
While ruminating, at a conference she had a chance encounter with a fellow researcher, Emmanuel Charpentier, whose lab was on the brink of discovering that one CRISPER flavor, the Cas-9 flavor, “… is an amazing enzyme that can recognize the DNA at sites that match … a twenty letter sequence in the guide RNA.”
By then, scientists had realized that the CRISPER-Cas immune system comes in two classes. Class 1 requires multiple proteins to perform its immune response function, while the Class 2 systems each consist of “… a single large gene that encodes a big protein …” that protects the cell.
Working with Charpentier, Doudna investigated the function of the gene encoding the strange and powerful protein known as Cas 9, the “…one protein” she said while waiving her hands, that “… does everything.”
Together they figured out that Cas 9 has the ability to recognize segments of DNA at sites that match a twenty “…letter sequence in the guide RNA and remember that this would be an RNA molecule coming from and derived from integrated pieces of DNA in the CRISPER locus that record a past viral infection so by definition these RNA are able to recognize matching DNA’s that come from those viruses.”
The protein is able to unwind the DNA duplex, then generate “…a break in the double stranded DNA.” Through the work in Doudna’s lab, scientists are now able to use this system to delete genetic material from human DNA, as well as to insert new genetic material into both somatic (any biological cell from any organism) and germ-line cells (cells that pass on their genetic material.)
Doudna’s cartoons (her chosen term) are clear, her words rise and fall over the hills of a wonderful journey. But after almost twenty years in and around the life sciences industry I have come to appreciate the limits of my ability to understand a good life science story.
__________________
True to her word, Doudna left ample time for questions. Many centered on the same themes – how things could go wrong. However, one seemingly elderly questioner (off camera) was very particular. He wanted to know whether the tool could be used to replace the faulty gene that results in macular degeneration and if so, where would scientists find the new, working gene.
For all of these sorts of questions, Doudna offered professional patience and a knowing smile. Audience members were full of “… good questions…” almost all of which require us “…to think hard.” But thankfully, a lot of her colleagues are already thinking hard, along with various regulators.
Toward the end of her talk, one questioner, in halting English, inquired as to her thoughts on whether the “ …VC’s gushing interest in this area was a positive or negative thing.”
This question “…touched on a very important point…” Doudna responded, one that she hadn’t given enough time to in her talk. Yes, she acknowledged, “…there’s tremendous interest from the biotech and investor communities that will enable all sorts of commercial activities that will lead to important advances.” But there will also be conflicts between the research and business opportunities of those companies – “…things which will need close attention and transparency.”
Doudna was careful to mention the “…very exciting ways of advancing technology that involves partnering with companies and this is something that I really didn’t know anything about until a few years ago …my work never had any commercial implications what so ever.”
Now it seems she’s up to speed as, just after the formal talk had ended and before the questions began, Douda profiled her commercial experience: She is a founder of four biotech companies, a strategic board advisor to three additional companies and a member of the Board of Directors of Johnson & Johnson, by some measures, the world’s largest bio-pharmaceutical company.
Fortunately, she’s benefited “…from experts at Cal …” who have spoken with her about different kinds of partnerships. “But,” she added, “ I think there are times when research … in academic labs … has potential commercially…” but for various reasons “…really can’t be explored by an academic lab because we don’t have the resources and frankly because we don’t have the desire or the expertise to take the work in that kind of direction – so by partnering we can do things together that neither of us can do alone.”
The eighth question came from a gentleman, again speaking off camera, in one of those voices that comes in after the battle scene in a Ken Burns documentary. This questioner wanted to “… touch on eugenics … given the dark history … In particular…,” the questioner wondered, “… do you envision the government taking a harsh stance early on, …” or do you see the challenge as one that will be “…left to the market place.”
“Well I guess,” answers Doudna,
… I imagine it’s likely to move forward in the way that in-vitro fertilization has unfolded. So I’m old enough to remember when my parents would sit at the dinner table and debate the morality of test tube babies ya know and (making faces to convey how fraught these issues were) talk about was it right for people to be conceived in a test tube and ya know it seemed really weird but then over time we had family friends who benefited from IVF …
… and so over time in a very grass roots way people came to accept that technology and I almost wonder if we’ll see a similar thing with human germ-line editing that perhaps it will start to be used in some IVF clinics … then if the those uses result in perceived benefits to kids and to families then you could imagine that will start to be more widely adopted.
…now does that mean we’re likely to be entering into a period of eugenics …I, I, I don’t see that happening … it’s likely to be more sporadicly utilized and I would hope that initial uses would be limited to really medical need rather than what we might consider to be enhancements.
__________________
At this point in the talk I was reminded of an article that I had read almost fifteen years ago, which in turn brought me back to a conversation that I had had several years prior.
I was flying from London to New York, back when seats were comfortable. Back when the experience of riding at 35,000 feet above the Atlantic in pure-pitch black, opened travelers to wonder, stripped veneers, randomly turning some into listeners, others to talkers.
That night, as the wine poured, I became a listener. My seat mate was a young English tech executive on his was to do something in New York. I, a moderately young life sciences executive (though in full discloser, on the finance side of things) back from visiting the new R&D facilities in Sandwich, facilities that would be shuttered not long after the paint dried.
My companion told me this: His wife had just given birth. But, congratulations weren’t in order. His new child, the second in the family, was born severely premature, then a relatively common occurrence with IVF.
Medicine has cures to treat the symptoms of its marvels. The doctors rushed in and miraculously saved that baby’s life. Which would have been good except that the first child, then just under two, also an IVF-child, having arrived so frighteningly early, tested the bounds of miraculous intervention, such that what was saved was severely developmentally disabled. Severely.
“If we had known,” my seat mate said, “if we had known.” He was alone in his guilt in a way that only a first mover can experience.
I found the article that had caused me to think back on that conversation. It appeared in the February 2006 edition of the Harvard Business Review. Debora Spar, then President of Bard, argues that the best solution to regulating usage of the then still relatively new technology of IVF to meet the demands from the wealthy who desire to procure offspring, is to acknowledge that the course of the technology would be best driven by market forces.
Trying in other ways to shunt progress or restrict access will, Spar reasoned, lead to less desirable outcomes. The demand won’t go away; any restrictions will be subverted with consequences worse than whatever open access would result in.
But to make a market, Spar writes, requires assignable property rights: Something that can be defined, then encoded in law, thereby allowing growth to be within rails.
“Some lament the very existence of this baby trade, insisting that reproduction—like love or honor—should never be sold …” But the absence of a market with its well defined property rights, reveals ”… an unfortunate unwillingness on society’s part to wrestle rules of nature.”
However, Spar notes, “Defining these rights would not resolve the deep moral issues that this market raises.” A system of property rights would be “ …a vital intermediate step …” but would not “ … tell us which pieces of this emerging technology are acceptable or for whom.”
“A far better approach,” Spar adds,
… would be for Americans to decide, as a society, just what we consider acceptable in the baby trade. Are we comfortable allowing commercial exchange in the pursuit of procreation? Are we willing to permit parents and their doctors to manipulate the embryos that will become their offspring? How will we determine which procedures push the trade too far? Any one person—this author included—is likely to have strong views on each of these questions. But the process here is far more important than any single set of conclusions. Americans need to debate these questions. For without such a process, we will never be able to arrive at a regulatory strategy that sticks.”
Doudna, to the best of my understanding, is not suggesting that some form of property rights be assigned to facilitate a market for choice-driven gene editing. But just how capable are we – is society – of wrestling these issues, now coming one after another. And even if we were to wrestle them, where would that take us?
In the old days, first there was science, then after many years came the technology. In the new world of bio-tech, the technology is the science, leaving little space for reflection.
__________________
Toward the end of her prepared remarks, Dr. Doudna asks a rhetorical question: What about editing the human germ-line? That is, instead of inserting genetic material into a fully developed human cell, what about an edit or insertion of new DNA into a germ-line cell such that that particular edit will remain in the DNA “ … so that [it] can be passed on to future generations.”
In November of 2019, Dr Doudna received an email from Jua Juan who had “ … visited Berkeley a couple of times …” Jua Juan is not an MD and though he had not been directly involved in the research that lead to the ability to manipulate the CRISPER Cas-9 system, because the technology is “ …not that difficult …” Jua Juan was “ …able to find various partners …” who helped him implant into the germ-line of infant twins “ … changes that have never been tested.”
Through a twitter feed, a professor at the University of Massachusetts published a careful analysis “ … of the evidence.” That feed revealed that although the stated purpose was to remove the CCR5 receptor for the HIV virus, “ … the actual edits were changes that have never been seen in humans …”
The response from the global scientific community was swift. The response inside China, severe: Jua Juan was sentenced to three years in prison. But what do we know about this sentence? Did Jua violate a law? Or did he prematurely reveal ongoing efforts to explore the CRISPER Cas-9 system?
__________________
Braden Allenby writes about the challenges facing our regulatory infrastructure, how the slow grind of public discourse is no match for the pace of science, and how the yawning gap between a regulatory body and the issues it’s tackling is likely never to close.
Allenby arrives at a MAD-like thesis:
Cultures that attempt to block technology for reasons that appear desirable will, all things equal, eventually be dominated by those that embrace it. This obviously poses an unhappy dilemma: if a culture wishes to maintain dominance, must it develop all technologies where it is capable of so doing? If this is the case, does it imply that ethical judgments about technologies move over time to the lowest common denominator? There are no good answers to these questions, but they do indicate the likelihood that in a highly competitive global environment, where many cultures are jostling for position, technological evolution will be difficult, if not impossible, to stop.
References:
Dr. Jennifer Doudna discusses gene-editing at the Institute for International Students, UC Berkeley: https://youtu.be/9Yblg9wDHZA
Where Babies Come From: Supply and Demand in an Infant Marketplace, by Debora Spar, HBR, February 2006 Issue
Innovative Governance Models for Emerging Technologies, Braden Allenby, et al., Edward Elgar, 2013. (pg. 33)
