Borko Amulic and Gabriel Sollberger in The Scientist:
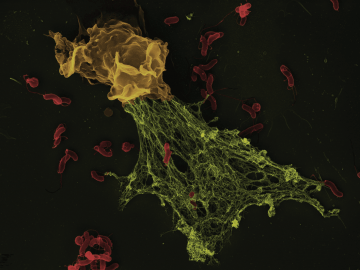 In the early 2000s, Arturo Zychlinsky at the Max Planck Institute for Infection Biology in Berlin found that mammalian immune cells called neutrophils use an enzyme called neutrophil elastase (NE) to cleave bacterial virulence factors. When Zychlinsky and his colleagues delved deeper into this defense mechanism, they realized that when activated by bacteria, human neutrophils release NE in what, under the microscope, looked like a fibrous structure. This structure turned out to be a meshwork of NE, other proteins, and copious amounts of DNA. In cultured human neutrophils, the webs were able to trap the bacteria that had triggered their formation, thereby limiting infection, so Zychlinsky and colleagues dubbed them neutrophil extracellular traps, or NETs.
In the early 2000s, Arturo Zychlinsky at the Max Planck Institute for Infection Biology in Berlin found that mammalian immune cells called neutrophils use an enzyme called neutrophil elastase (NE) to cleave bacterial virulence factors. When Zychlinsky and his colleagues delved deeper into this defense mechanism, they realized that when activated by bacteria, human neutrophils release NE in what, under the microscope, looked like a fibrous structure. This structure turned out to be a meshwork of NE, other proteins, and copious amounts of DNA. In cultured human neutrophils, the webs were able to trap the bacteria that had triggered their formation, thereby limiting infection, so Zychlinsky and colleagues dubbed them neutrophil extracellular traps, or NETs.
The fact that neutrophils used their nuclear material to catch pathogens was intriguing to immunologists and cell biologists alike. The work of the Zychlinsky lab suggested that the release of NETs was an active process, and that the material wasn’t simply released by passive lysis. This has motivated a new line of research devoted to characterizing these unique structures, delineating the mechanisms that prompt their formation, and identifying their relevance in mammalian biology. As more and more researchers join the burgeoning field, the spectrum of pathogens known to induce NET release from neutrophils has expanded from a variety of bacteria to fungi and, most recently, to viruses. However, it has also become clear that NETs can have negative consequences for the organisms that produce them—by activating autoimmune pathways or encouraging tumor cells to metastasize, for example.
More here.
