Hendriks and Besse in Nature:
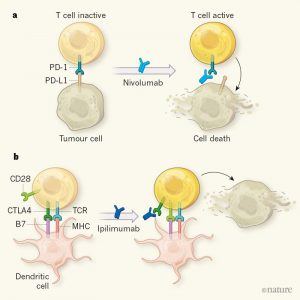 Chemotherapy became the standard treatment for lung cancer in the twentieth century1. But in the past 15 years, there has been a drive to improve outcomes for people with this still-deadly disease, either through therapies that target enzymes encoded by genes harbouring cancer-driving mutations, or through immunotherapies, which activate the body’s immune system to target tumours. Writing in The New England Journal of Medicine, two groups2,3 provide evidence that supports the use of immunotherapies to treat non-small-cell lung cancer (NSCLC) at different stages of the disease. Tumour cells evade destruction by activating signals known as immune checkpoints, which deactivate immune cells called T cells4. Two immune checkpoints are the proteins cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1), which are expressed by T cells themselves. Another, programmed cell death ligand 1 (PD-L1), is produced by tumour cells (Fig. 1).
Chemotherapy became the standard treatment for lung cancer in the twentieth century1. But in the past 15 years, there has been a drive to improve outcomes for people with this still-deadly disease, either through therapies that target enzymes encoded by genes harbouring cancer-driving mutations, or through immunotherapies, which activate the body’s immune system to target tumours. Writing in The New England Journal of Medicine, two groups2,3 provide evidence that supports the use of immunotherapies to treat non-small-cell lung cancer (NSCLC) at different stages of the disease. Tumour cells evade destruction by activating signals known as immune checkpoints, which deactivate immune cells called T cells4. Two immune checkpoints are the proteins cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1), which are expressed by T cells themselves. Another, programmed cell death ligand 1 (PD-L1), is produced by tumour cells (Fig. 1).
Antibodies that interact with these proteins to prevent their normal activity, and so reawaken the immune system, are now used to treat metastatic NSCLC — the stage at which the cancer has spread. Antibodies that bind PD-1 or PD-L1 are sometimes successful in patients who have had treatments such as chemotherapy, but whose cancer has nonetheless progressed5. Alternatively, the anti-PD-1 antibody pembrolizumab can be used as a first-line treatment for metastatic NSCLC when the percentage of tumour cells that express PD-L1 is high — these patients respond better to immunotherapy than to chemotherapy6.
If such immune-checkpoint-targeted antibodies (ICTs) can improve outcomes for metastatic NSCLC, could they also help to tackle early-stage disease? In the first of the current papers, Forde et al.2 carried out a pilot study to investigate whether the anti-PD-1 ICT nivolumab could be used to shrink tumours before surgery, which is a standard treatment for most cases of early-stage NSCLC. The authors treated 21 patients with 2 doses of nivolumab 2 weeks apart, starting 4 weeks before the planned surgery date. They showed that surgery did not need to be delayed (for example, because of an adverse event with nivolumab) for any patient. The researchers anticipated that four weeks would not be enough time for the reactivated immune system to significantly shrink the tumour. Indeed, imaging revealed significant shrinkage in tumours in only two patients before surgery. However, examination of the surgically removed tumours revealed that 45% had undergone a major response to the ICT — less than 10% of the tumour cells remained alive. ICTs, unlike chemotherapy, cause inflammation and scar-tissue formation in tumours, and can therefore sometimes cause tumour growth. However, the researchers found that even two tumours that showed such growth had undergone a strong pathological response.
More here.
